
Traditional Evidence Planning has long been a siloed activity, structured templates, sluggish spreadsheets, and cross-functional meetings where teams debated which studies, analyses, and publications would best support an asset’s journey.
At its core, Integrated Evidence Planning (IEP) aligns scientific, clinical, real-world, and value evidence to the decisions that matter most across the lifecycle: regulatory, clinical, payer, and patient.
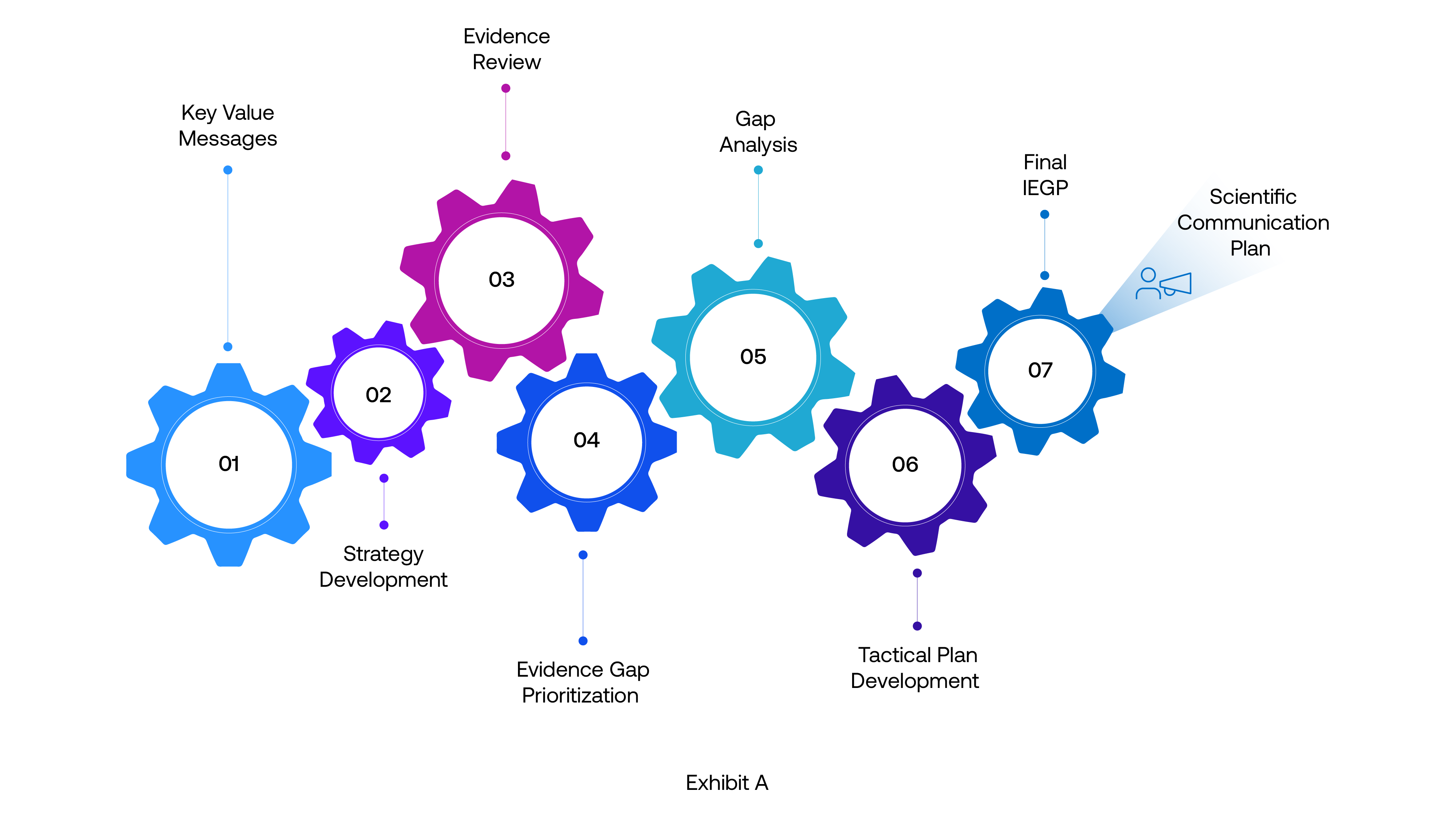
Let’s look at two scenarios that every IEP team recognizes
Scenario 1: The ‘we didn’t see that coming’ HTA outcome
An asset secures regulatory approval with strong efficacy. Then the HTA feedback arrives:
- Comparator not aligned with current clinical practice
- Outcomes not relevant to health-system cost drivers
None of these are surprises in hindsight, they were knowable.
Trends in HTA decisions, competitor endpoint strategies, and real-world treatment patterns already contained the signal.
In a predictive IEP model, signals like this trigger suggested evidence actions long before they become unmet expectations.
Scenario 2: The multi-system evidence hunt
A medical lead prepares for a high-stakes expert session and needs:
- Safety signals by subpopulation
- Competitive evidence sequencing
- Real-world switching patterns
- Most-cited claims in publications
- Unmet-need patterns from field insights
All of this exists, but it’s scattered across:
- Study portals and source datasets
- RWE portals
- Shared drives
- CRM systems
- Protocol repositories
- Publications/datavision
- Legacy excel trackers
Teams spend more time finding evidence than using it.
In the next phase of AI advancements in IEP, discovery becomes as simple as asking: “What is our evidence position versus Competitor A in Metabolic Disorder Type X?”
IEP has evolved, quietly but fundamentally
IEP is no longer just a planning exercise.
It is becoming the operating system for how science, medicine, and commercial strategy stay connected in one of the world’s most complex industries.
What both scenarios highlight is this. These are not failures of science, talent, or strategy, they’re failures of evidence orchestration.
What's driving the change
The first shift came when regulators worldwide began encouraging richer and more transparent evidence, accelerated approvals, real-world data guidance, and structured benefit–risk frameworks. Suddenly, teams weren’t planning evidence for single inflection points; they were architecting continuous evidence across the lifecycle.
Around the same time, payers changed the storyline. New reimbursement models, rising scrutiny on value, and increasingly competitive therapeutic classes meant that evidence couldn’t only be statistically sound, it had to be decision-ready, comparable, and clearly tied to outcomes that matter to real health systems.
Then, digitalization quietly accelerated everything. Publications ended up in reference managers. Study data landed in disconnected operational systems. RWE sat with different vendors. Medical insights lived in CRM tools. Protocols were authored in one platform, safety signals reviewed in another.
IEP became the only place where all this fractured information had to come together and make sense.
This fragmentation created a new reality: teams weren’t struggling with evidence generation, they were struggling with evidence orchestration.
And that is exactly where the next chapter begins.
Where AI steps in, not as a model, but as a partner
The challenge of modern IEP is no longer volume of evidence but the ability to:
- Detect patterns across clinical, RWE, medical insights, publications, and safety streams
- Connect decisions to the best evidence - instantly, regardless of source
- Predict, not just plan, the next evidence need across the lifecycle
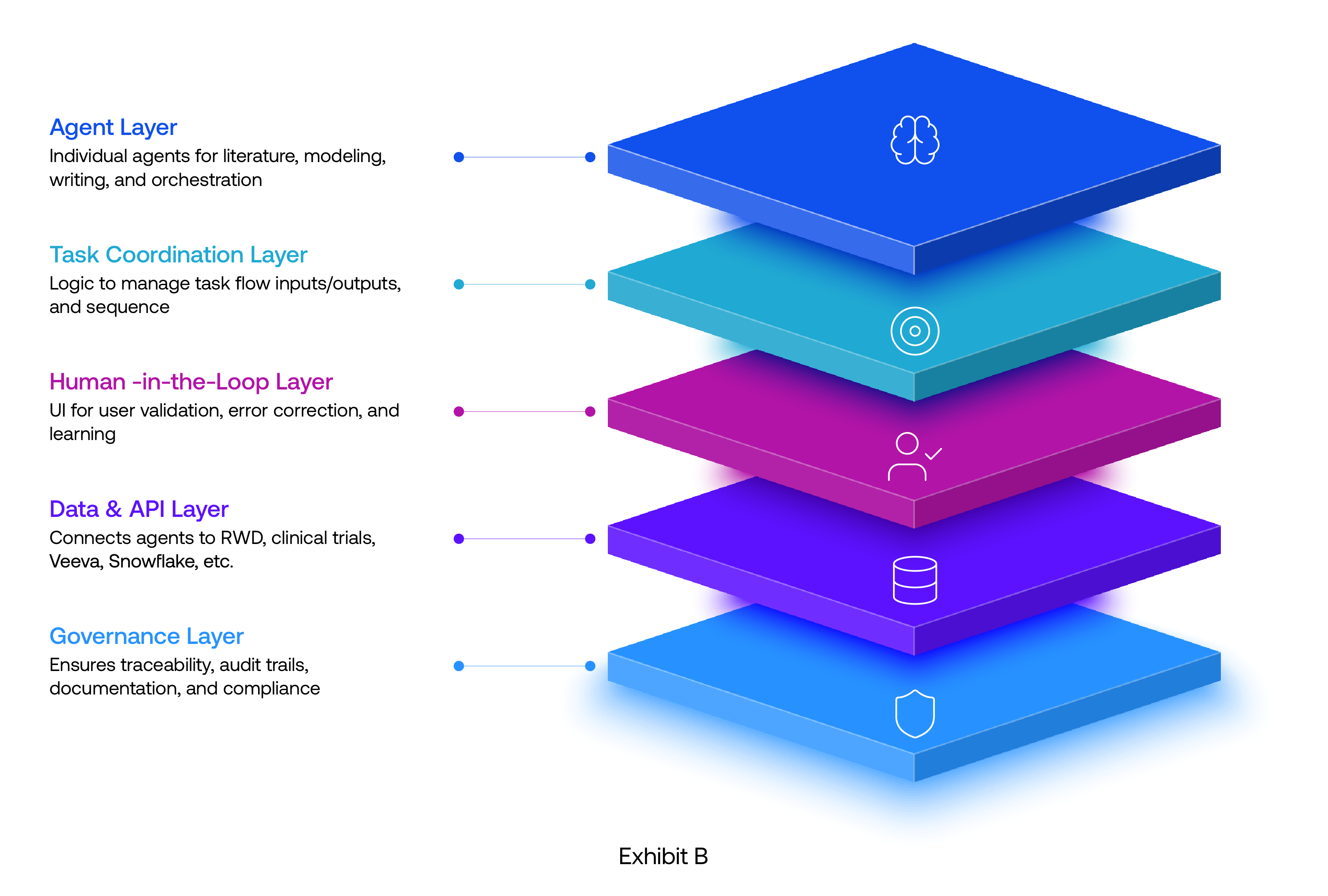
These needs are shaping a new class of AI capabilities inside IEP. The most transformative ones are emerging naturally from the pain points teams grapple with every day.
1. Evidence search and synthesis
Teams spend weeks combing through publications, protocols, SAPs, regulatory documents, and real-world studies to answer what should be simple questions.
- What evidence exists across subpopulations?
- What data gaps persist for payers?
- What endpoints have competitors used successfully?
- What recommendations did the last HTA make in this space?
AI is finally collapsing this search-to-insight burden.
Semantic and ontology-driven models can now read across all sources of evidence - clinical studies, RWE, HEOR, publications, medical insights, even structured fields inside planning tools, and produce a unified narrative or comparison.
Instead of navigating 15 systems, teams ask a question and get an instant synthesis. Evidence becomes discoverable, comparable, and explainable in a way the human eye alone could never achieve.
2. Predictive gap identification and scenario planning
Seeing around corners, not just looking back
As lifecycle evidence expectations rise, the biggest challenge is not knowing what to generate next.
AI can now look across analog assets, historical regulatory precedents, competing evidence landscapes, and payer decision patterns to suggest:
- Emerging evidence gaps that will matter
- Endpoints or populations competitors are likely to pursue
- Scenarios where new RWE could materially shift a payer’s view
- Elements of a study or analysis that would drive the highest downstream impact
This isn’t theoretical. These models are already grounded in real-world signals: past regulator questions, HTA trends, safety database patterns, unmet-need maps, and real-time shifts in clinical practice.
For the first time, IEP becomes predictive, not retrospective.
What’s next
The future of IEP isn’t another dashboard, static document, or repository.
It’s the shift from planning to continuous evidence intelligence.
Now, with AI acting as an Evidence Analyst colleague, evidence planning becomes dynamic, adaptive, and proactive where the shift isn’t just technological, it reflects the changing expectations of the industry.
- The need for consistent evidence logic and traceability
- Increased emphasis on regulatory transparency and defensibility
- Pressure to make faster, aligned decisions across clinical, medical, HEOR, and commercial teams
Visionet’s Evidence AI Analyst (powered by Flow AI* design principle)
Evidence AI analyst represents the next wave of IEP enabler where AI is not just a search tool, but an agent that moves work forward.
Your Evidence Analyst doesn’t wait for instructions. It:
- Surfaces gaps before they become risks
- Refreshes evidence strategy as new science or competitor actions emerge
- Suggests pathways, outlines impact, and facilitates alignment
It brings momentum into evidence planning, turning what used to be a reactive annual exercise into a living, adaptive workflow.
* Flow AI is a workflow-native AI orchestration layer, powered by reasoning agents that can discover information, interpret it, decide what matters, and take action across enterprise systems. It blends LLM reasoning, retrieval, and automation to create adaptive workflows that continuously learn and improve.




